Abstract
Mixed phenotype acute leukemia (MPAL) is a rare group of acute leukemias defined by immunophenotypic expression of more than one hematopoietic cell lineage. The World Health Organization (WHO) diagnostic immunophenotypic criteria for MPAL rely on the intensity of lineage-defining antigen expression, predominantly a qualitative assessment, and are often difficult to apply to a phenotypically heterogeneous leukemia. Cases of MPAL defined by isolated myeloperoxidase (isoMPO) expression on otherwise typical acute lymphoblastic leukemia (ALL) are variably diagnosed as MPAL or ALL based on the incompletely defined criteria for assigning MPO expression. We hypothesized that quantitative criteria for antigen intensity could be developed and applied in a uniform manner across flow cytometry instruments, reagents, and analysis software to enable a consistent approach to diagnosing MPAL and better defining isoMPO.
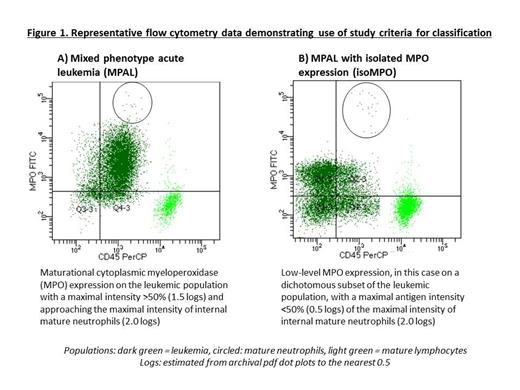
We previously reported on a multicenter cohort identified by respective institutions as MPAL with subsequent central review according to 2008 WHO criteria (Oberley et al 2020). Of these, 100 had suitable dot plots for reevaluation (89: B/Myeloid, 10: T/Myeloid, 1: B/T). Antigen expression was concurrently reviewed by two hematopathologists to reach consensus (BLW, AEK). The cohort was divided a priori into randomly selected training and testing cases (n=30/n=70). Classification criteria were generated in the training cohort for WHO lineage-defining antigen expression (myeloid: cMPO, CD64, CD14; B: CD19, T: cCD3) from 10 cases and then refined through review of an additional 20 cases. Positive antigen expression was classified as maximal intensity approaching that of the mature normal counterpart population (NCP) (cMPO: neutrophils; CD64, CD14 and CD11c: monocytes; CD19: B cells; cCD3: T cells) either by 1) range of expression recapitulating maturation of the NCP, irrespective of the percentage on the leukemic population (Figure 1A); or 2) uniform expression above background on a discrete (sub)population (Figure 1B). To account for technical variation within and among laboratories, maximal antigen intensity on the leukemic population was measured in 0.5 log increments and normalized to the maximal intensity of the NCP. An intensity of ≥50% of the NCP above background was defined as sufficient for MPAL lineage assignment and <50% consistent with isoMPO. This approach was then used to classify the remaining 70 cases.
Using this approach, 41/98 (42%) cases previously classified as MPAL remained classified as MPAL: 31 as B/Myeloid (25 by maturational MPO expression, 6 by maturational CD64 and/or CD14 expression); 9 as T/Myeloid (8 by maturational MPO expression, 1 with maturational CD64, CD14 and CD11c expression); and 1 as B/T. No cases in the cohort showed uniform expression ≥50% of the NCP. The remaining 57 showed isolated low-level MPO expression (maximal intensity <50% of the NPC) on a uniform leukemic population or on a dichotomous subpopulation (isoMPO), 56 B/Myeloid and 1 T/Myeloid. Two cases of otherwise typical B-ALL without myeloid or monocytic antigen expression were reclassified as B-ALL, one of which showed low variable CD10 suggestive of DUX4-rearranged B-ALL. In comparison to our previously reported study of 2008 WHO-defined MPAL, 53/89 (60%) centrally-confirmed cases would be classified here as isoMPO. Conversely, five cases excluded under 2008 WHO central review would be reclassified as having sufficient antigen expression to qualify as MPAL (2 B/Myeloid, 3 T/Myeloid).
Novel semiquantitative immunophenotypic criteria applied to a large cohort of acute leukemias originally diagnosed as MPAL was able to objectively identify a large subset as having dim, uniform MPO expression (isoMPO). Our approach emphasizes antigen expression recapitulating maturational expression similar to their non-leukemic cellular counterparts, normalizes absolute intensities to internal positive and negative control populations to minimize technical variability between observers and assays, and as per the 2017 WHO, does not require a specific percent threshold of positivity. While requiring validation, this is a critical first step toward establishing a reproducible delineation of these complex cases to practically implement the WHO classification to support treatment decisions and ongoing investigation into MPAL genomics and outcomes (available for this cohort by ASH).
Oberley: Caris LIfe Science: Current Employment. Orgel: Jazz Pharmaceuticals: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal